Pharma’s QC lab of the future: Boosting speed, compliance, and quality
Biopharma executives report measurable gains from quality control lab modernization
Dr. Laks Pernenkil
Iknam Gill
Laurent Oliver Becher
Prateek Saini
Wendell Miranda
Apoorva Singh
Imagine a quality control lab that anticipates and prevents issues, where operators access personalized tasks and insights via biometric logins, and sample preparation is fully automated. Extended reality tools guide operators through testing procedures, while instruments stream real-time data to the cloud. Artificial intelligence agents accelerate validation and batch release, flag deviations in real time, identify likely root causes, and recommend next-best actions to operators. These advances could mean faster time-to-market, fewer compliance concerns, more time to focus on complex problem-solving, and higher, more consistent quality.
The quality control (QC) lab of the future appears to be emerging in the biopharma industry as an intelligent, agile, and highly automated environment powered by AI, robotics, and cloud computing. According to a 2025 Deloitte Center for Health Solutions survey of 103 biopharma executives, modernization efforts are underway (see methodology), driven by the need to accelerate time-to-market, adapt to shifting regulations and policies, and meet increasing quality standards.1 Consistent execution across manufacturing, supply chains, and especially quality control is now important for successful drug launches.2 As global quality organizations within biopharma companies pursue holistic digital transformation, QC labs have a distinct opportunity to incorporate digital tools and data-driven approaches to enhance operational performance.3
Investments in lab transformation already appear to be delivering results: 50% of survey respondents reported fewer errors and deviations, 45% noted improved compliance, and 43% observed shorter testing timelines. Furthermore, integrating QC lab insights with research and development, using unified laboratory information management systems, digitized platforms, and AI could streamline method development and transfer. This could help accelerate scale-up and improve the likelihood of achieving quality on the first attempt.4 However, 40% of respondents said their QC labs still operate with disconnected systems and limited automation, highlighting a gap between current practices and digital potential.
Companies that delay lab modernization could face longer product release cycles, higher error rates, and weaker regulatory readiness. More than half (56%) of the executives surveyed said their organizations could achieve more automated and predictive lab capabilities within the next two to three years. In general, respondents were optimistic about the potential impact of digitization and automation, projecting that it could lead to a 20% to 50% reduction in compliance issues, a 15% to 30% decrease in operational costs, and a 20% to 30% improvement in scale-up speed. However, it is important to note that these figures reflect executive forecasts for future performance—not actual outcomes. The extent to which these benefits are achieved will likely depend on each company’s current level of digital maturity, its investments in lab modernization, and the effectiveness of its transformation strategies.
Although individual results may vary, the overall trend seems clear: Modern QC labs have the potential to become strategic engines of efficiency, resilience, and accelerated time-to-market. To help them fully realize the benefits of lab modernization, biopharma companies can consider approaching it as an ongoing strategic journey rather than a one-time upgrade. Effective modernization will likely depend on many factors, including:
- Defining a clear, shared vision of the future lab
- Identifying and prioritizing critical capabilities
- Establishing an agile execution model
- Tracking outcomes using defined key performance indicators
- Embedding culture change and ensuring workforce readiness across the journey
QC lab modernization is delivering tangible results and fueling ongoing momentum
According to findings from the Deloitte 2025 QC Lab of the Future Survey, many companies are prioritizing investments in robotic automation, instrument connectivity, AI, and generative AI. These technologies are being implemented to automate data capture and sample management, optimize asset and resource utilization, and enhance end-to-end traceability of samples, results, and processes. Looking ahead, agentic AI systems that can sense, reason, and act could enable intelligent QC workflows. Such systems could support real-time monitoring, early anomaly detection, and dynamic resource allocation, further enhancing the efficiency and effectiveness of laboratory operations.5
Survey results indicate that biopharma companies are investing in lab modernization to varying degrees, and this trend is likely to continue (figure 1). In the next two to three years, at least 70% of surveyed executives—representing companies of different revenue sizes—said their company plans to maintain or increase their investments in QC lab modernization. This ongoing commitment likely reflects growing confidence in the benefits of modernization and highlights its role as a catalyst for operational excellence and competitive advantage.
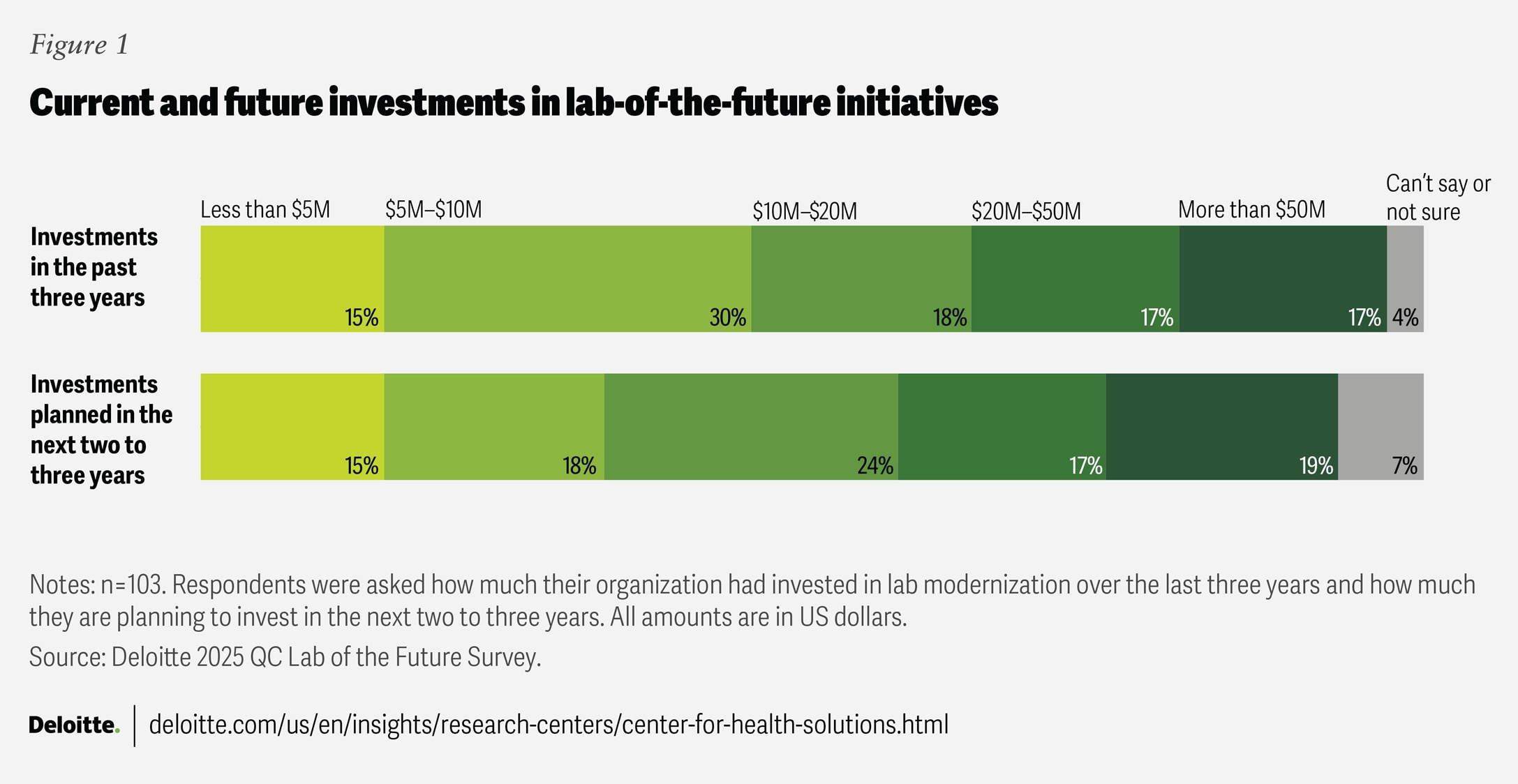
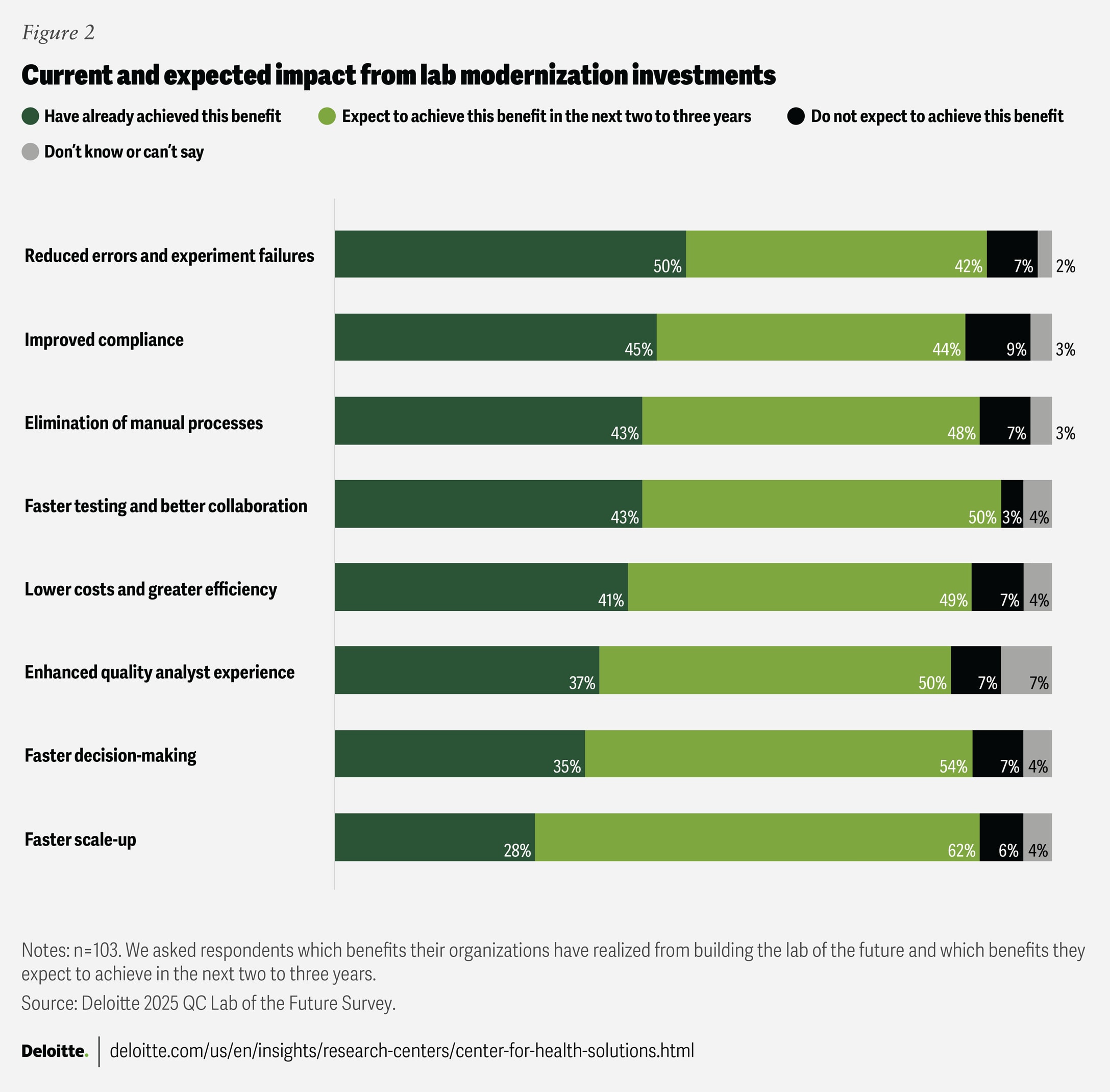
As noted earlier, lab modernization efforts are beginning to deliver operational improvements, such as reduced error rates, enhanced compliance, and faster testing timelines (figure 2). Still, more than half of surveyed executives reported that their organizations have not yet realized these benefits, underscoring that transformation tends to be an ongoing journey rather than a quick fix.
Looking ahead, there appears to be a sense of optimism among the surveyed executives about the potential impact of lab modernization over the next two to three years. Sixty-two percent expect faster scale-up as predictive analytics and automation streamline processes such as method transfer, equipment qualification, and batch release. Fifty-six percent anticipate faster, data-driven decision-making, while 50% foresee an improved operator experience as automation reduces repetitive tasks and AI-enabled validation minimizes rework. Realizing these benefits could enable biopharma companies to shift from reactive quality management to proactive, intelligent quality control.
QC labs are advancing toward automated and predictive operations
As lab-of-the-future initiatives progress, QC labs are likely to evolve along a digital maturity curve—from digitally nascent environments toward fully integrated, automated, and predictive operations. This journey is not uniform: Survey data reveals that QC labs fall into six distinct maturity levels (figure 3), and digital maturity can vary widely even within the same company, particularly in large multinationals.
Here’s an example of this variation from the survey findings: 40% of executives said their labs are “digitally siloed,” with fragmented data spread across systems, such as laboratory information management systems, electronic laboratory notebooks, and individual instruments. These labs also have limited automation of processes and workflows. Meanwhile, 30% of executives said their labs have reached a “connected” stage, with systems partially integrated and some automated lab processes in place. However, some labs that have reached a “connected” stage today may still struggle with inconsistent data sharing, making it challenging to monitor instrument health, resolve issues proactively, and continuously improve quality.6
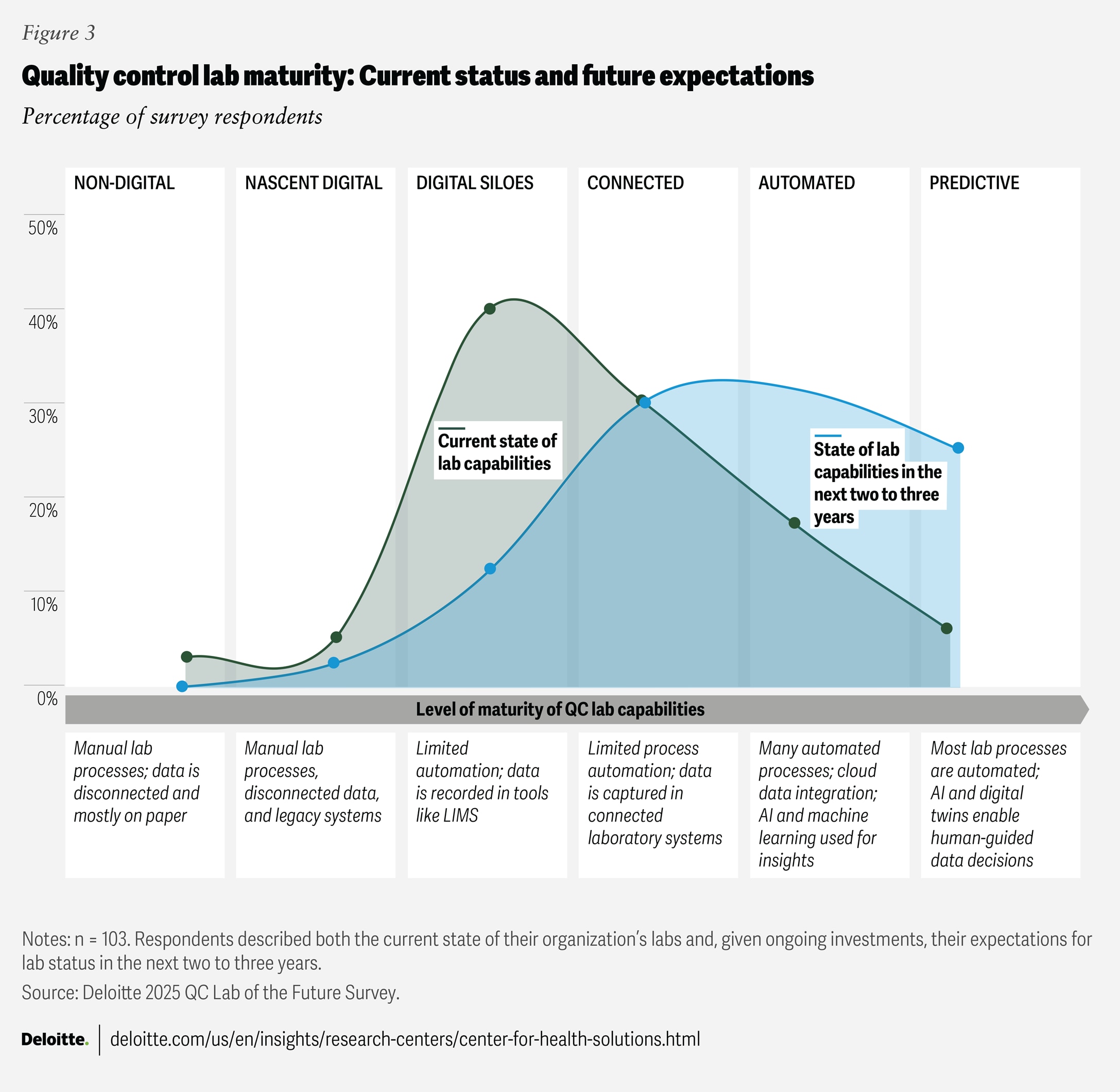
Looking ahead, surveyed executives believe that lab-of-the-future initiatives could transform QC lab capabilities. Over the next two to three years, 38% expect their companies to automate many processes and embed AI and machine learning into their quality control operations. Many companies have already started using automation solutions, AI, machine learning, and gen AI across various processes. More than 50% of the executives surveyed said their companies are either scaling up or implementing these technologies in areas like method life cycle management, sample management, and knowledge management.
When companies fully integrate automation and AI-driven systems that support continuous learning and real-time process adjustments, they can achieve a “predictive” level of lab maturity where quality control is optimized. In these advanced labs, AI agents can independently oversee, analyze, and report on processes, helping reduce inspection gaps, quickly detect anomalies, and enhance compliance. These agents can record every action and data point, from sample collection to final release, creating a transparent audit trail. Currently, only 6% of respondents indicated that their companies’ labs have reached this level of maturity, but 25% believe it is achievable within the next two to three years.
As companies advance their QC labs along the maturity curve (figure 3), they are likely to encounter multiple challenges, including:
- Poor interoperability: Forty-five percent of respondents identified poor integration between lab systems and instruments as a top obstacle. This tends to create fragmented data environments and limits automation, standardization, and adoption of AI and predictive analytics.
- Competing priorities: High upfront costs associated with automation, instrumentation upgrades, and workforce reskilling may lead companies to deprioritize lab transformation in favor of more immediate business needs. This is reflected in the survey data, with 38% of respondents citing competing priorities as a major challenge to modernization efforts.
- Lack of sustained executive commitment: Without clear, ongoing executive commitment, lab modernization efforts may struggle to gain traction and secure ongoing funding, potentially stalling execution or reducing returns. Thirty-eight percent of survey respondents identified securing leadership support as a top-three challenge.
Maximizing the impact of lab-of-the-future initiatives
To help unlock the full potential of lab modernization, biopharma companies should consider approaching transformation with structured intent, agile execution, and a sustained focus on measurable outcomes. High-achieving lab-of-the-future programs are driven not by technology alone, but by deliberate choices that span strategy, capability, and culture. Building on these ideas, companies can take several practical steps to accelerate lab modernization and realize its full benefits.
Define a clear, shared vision of the future lab
To help drive meaningful outcomes, companies can establish shared, future-state intentions for their lab modernization program. This involves aligning stakeholders—from site-level teams and managers to senior leadership—on what the next-generation lab should enable in terms of speed, scalability, and regulatory performance. Early alignment can help prevent missteps that hinder execution or dilute impact. It also involves a nuanced understanding of site-level needs and local ways of working, which may differ from broader leadership expectations.
When undertaking lab modernization, companies might consider different transformation paths depending on their urgency to change, appetite for risk, and available resources. A gradual path can enable incremental improvements, for example, automating sample tracking over several years. An intentional path follows a structured, phased roadmap, such as a two-year plan to digitize all lab workflows. A leapfrog path is intended to accelerate change by adopting advanced digital capabilities from the start, for instance, by immediately deploying cloud-based, AI-driven analytics across all labs. Selecting the appropriate path helps align planning, resource allocation, and the pace of change.
Identify and prioritize critical capabilities
With vision and alignment in place, companies may consider focusing on identifying the capabilities needed to bring that vision to life. Assessing current maturity across lab systems, data flows, automation readiness, and workforce skills can help uncover gaps. This diagnostic approach is intended to enable companies to prioritize investments in use cases that could have the highest impact. These can include automated high-throughput screening, integrated laboratory information management systems and electronic laboratory notebook platforms, advanced analytics for quality control, or AI-driven assay optimization.
Lab-of-the-future initiatives involve a balance between standardizing core processes and allowing differentiation at the edges. For instance, before developing differentiated capabilities such as digital twins to simulate method development companies should first consider establishing standardized lab processes, harmonized systems, and integrated data flows. These foundational capabilities are important to help enable scalable, interoperable, and sustainable transformation across the lab network.
Establish an agile execution model
Once priorities are defined, companies can move from planning to action. Agile delivery models such as product-oriented delivery (POD) teams can enable rapid iteration, stakeholder engagement, and early wins. PODs can also help test capabilities at pilot sites, refine solutions, and scale more effectively across labs. While agile execution emphasizes flexibility and speed, it tends to be most effective when grounded in a clear and structured roadmap. Over 70% of survey respondents who reported faster time-to-market, cost efficiencies, and improved compliance also indicated they had a clear roadmap in place to guide their transformation efforts.
Track outcomes using defined key performance indicators
As capabilities are deployed, companies should measure and clearly communicate the value delivered. However, this appears to remain a challenge for some companies, as 32% of survey respondents identified demonstrating return on investment as one of the top three obstacles in lab modernization efforts.
Establishing outcome-driven KPIs from the outset can help sustain momentum, secure ongoing investment, and guide course corrections. These KPIs could track both operational outcomes (such as throughput, downtime, equipment use, analyst productivity, and cost savings) and user experience factors (like adoption, satisfaction, training completion, and support needs).
Embed culture change and ensure workforce readiness across the journey
Culture change should not be treated as a bolt-on; it should be embedded in every phase of transformation. If culture is left unaddressed, resistance to change and reliance on legacy systems could all slow the adoption of new technologies and ways of working. To help them overcome these challenges, companies should consider establishing a clear vision, articulating a compelling case for change, and giving end users visibility into validation processes. This can help ensure that employees understand how compliance will be safeguarded and feel confident supporting the transformation.
As execution progresses, it’s important to consider embedding training, communication, and adoption support into both POD delivery and KPI tracking. By doing so, companies can strengthen the use of new technologies and tools. When lab operators and analysts are equipped, engaged, and empowered to adopt new ways of working, transformation efforts are more likely to deliver lasting impact and be scaled across multiple sites.
Taken together, these five considerations offer a structured approach for scaling lab-of-the-future initiatives. When applied consistently, they can help biopharma companies convert transformation ambitions into measurable, enterprise-wide performance improvements.
Biopharma lab modernization transforms strategy, accelerates speed, and elevates quality
Lab modernization is no longer just a technical exercise; it represents a strategic shift that can strengthen quality, shorten release cycle times, and help companies keep pace with regulatory and scientific complexity.7
Some biopharma companies are beginning to move beyond the question of whether to modernize, focusing instead on how to do so with precision, speed, and sustained impact. Survey respondents noted progress across several areas, from aligning leadership and investing in foundational capabilities to embedding changes across systems and workflows. While only a small share of labs currently operates at a predictive or AI-augmented level, 25% of surveyed leaders believe this is achievable within the next two to three years.
The opportunity seems clear, but execution appears to remain a challenge. Companies that move with urgency and discipline, anchoring their efforts in shared intent, measurable outcomes, and agile delivery, could transform quality control labs into engines of resilience and strategic value.
Methodology
This analysis is based on survey responses from 103 biopharma executives collected in April 2025. Respondents were involved in quality control operations across a diverse mix of biopharma organizations, ranging from early-stage startups to large, well-established companies. Roles represented include vice presidents, senior vice presidents, assistant vice presidents, senior scientists, directors, senior directors, and other executives involved in QC operations.
The survey aimed to evaluate investment trends in lab modernization, adoption of digital technologies, realized value and ongoing challenges, benefits of digital investments for QC lab operations, and expected impact on lab productivity.
For this survey, lab modernization was defined as the use of robotics, advanced analytics, cloud-based data management, connected lab instruments, AI, and other digital technologies to enhance efficiency, collaboration, compliance, and make lab operations more predictive and automated.
Note: The survey sample may not be fully representative of the entire biopharma industry and reflects the perspectives of executives involved in QC operations at the time of data collection.