Introduction to the Clinical Trials Regulation (536/2014) has been saved

Blog
Introduction to the Clinical Trials Regulation (536/2014)
The European Commission hopes to create a favourable environment to conduct clinical trials in the EU with the implementation of the Clinical Trials Regulation (CTR), which is expected to go live at the beginning of 2022. This blog outlines the key challenges organisations may face in order to be compliant with the new Regulation and become more responsible businesses.
By Paulien Nuyts, James Biddlecombe and Sebastian Payne
Go directly to
- Background
- Directive vs. Regulation
- Clinical Trials Information System (CTIS)
- Timeline
- Relevance for organisations
Background
The European Commission hopes to realise its ambition to create a favourable environment to conduct clinical trials within the EU with the implementation of the Clinical Trials Regulation (CTR) (No 536/2014). The CTR is the new regulation that in the near future will need to be adhered to for all interventional clinical trials conducted in the EU with medicinal products for human use. It aims to harmonise submission and assessment processes, improve cooperation and transparency in and between Member States and enhance overall safety standards. Throughout a series of blogs we will examine key changes and challenges that organisations may face as a result of the introduction of the CTR, as well as the potential competitive benefits for organisations. In this first blog, we broadly examine the changes between the Clinical Trials Directive 2001/20/EC (Directive) and the CTR and what this could mean for becoming more responsible organisations.
Directive vs. Regulation
Before 2004, processes and requirements for clinical trials were determined at the Member State level and therefore varied widely across EU countries. In May 2004, the Directive was adopted, which was the first step towards harmonization of the clinical trial processes and requirements. However, under the Directive the application of clinical trials remains highly fragmented. An example is clinical trials involving more than one Member State (still) require separate applications to be submitted to each country.
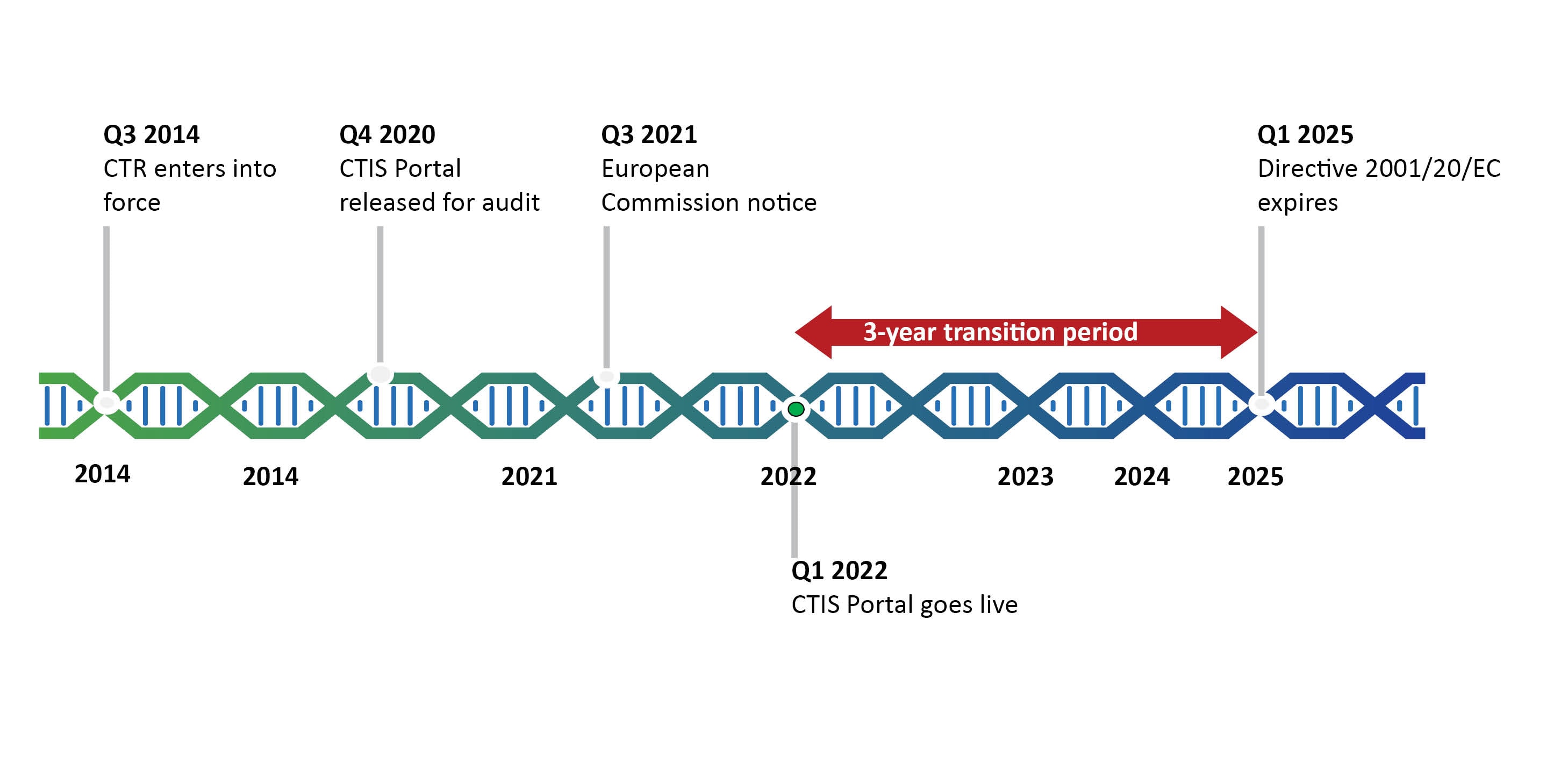
The Directive was the first step towards harmonization and the CTR takes this a step further by creating a single-entry point for clinical trials in Europe (see Figure 1). Although adopted in 2014, the Clinical Trials Regulation is yet to go into force and is currently expected to go live at the beginning of 2022. This is determined of the go live date of the parallel designed Clinical Trials Information System (CTIS).

Clinical Trials Information System (CTIS)
The CTR introduces the CTIS portal that will allow all clinical trial applications to be submitted through one single system. The introduction of such a portal will simplify the application process. Using this system, sponsors submitting applications to multiple MSC will be able to submit a single application via the system to all countries at once. Under CTR, all MSC will provide a joint assessment and a single decision of Part I of the application, which covers general trial, sponsor and product information. More information on submissions strategies under CTR will be discussed throughout this blog series.
In addition to changes in the application process, the CTIS system allows more data to become publicly available. The European Medicines Agency (EMA) publishes documents uploaded to the portal, thereby increasing transparency of clinical trial information.
Timeline
The timeline of the Clinical Trials Regulation is challenging due to the required development and go-live of the CTIS. The CTR entered into force on 16 June 2014, but is expected to become effective in the 1st Quarter of 2022 due to unexpected delays in the technical development of the CTIS. The go-live of the CTIS is dependent on the successful passing of the independent audit expected to be performed in December 2020.
Should the audit be passed and the system go live according to the current schedule, from Q1 2022 a transition period will start until Q1 2025. The transition period will give sponsors opportunities to make use of the CTD and the CTR interchangeably. These opportunities will be outlined further in this blog series.
*In the recent DIA CTR Conference, the EMA suggested that CTIS may go live at the end of the year 2021.
Relevance for organisations
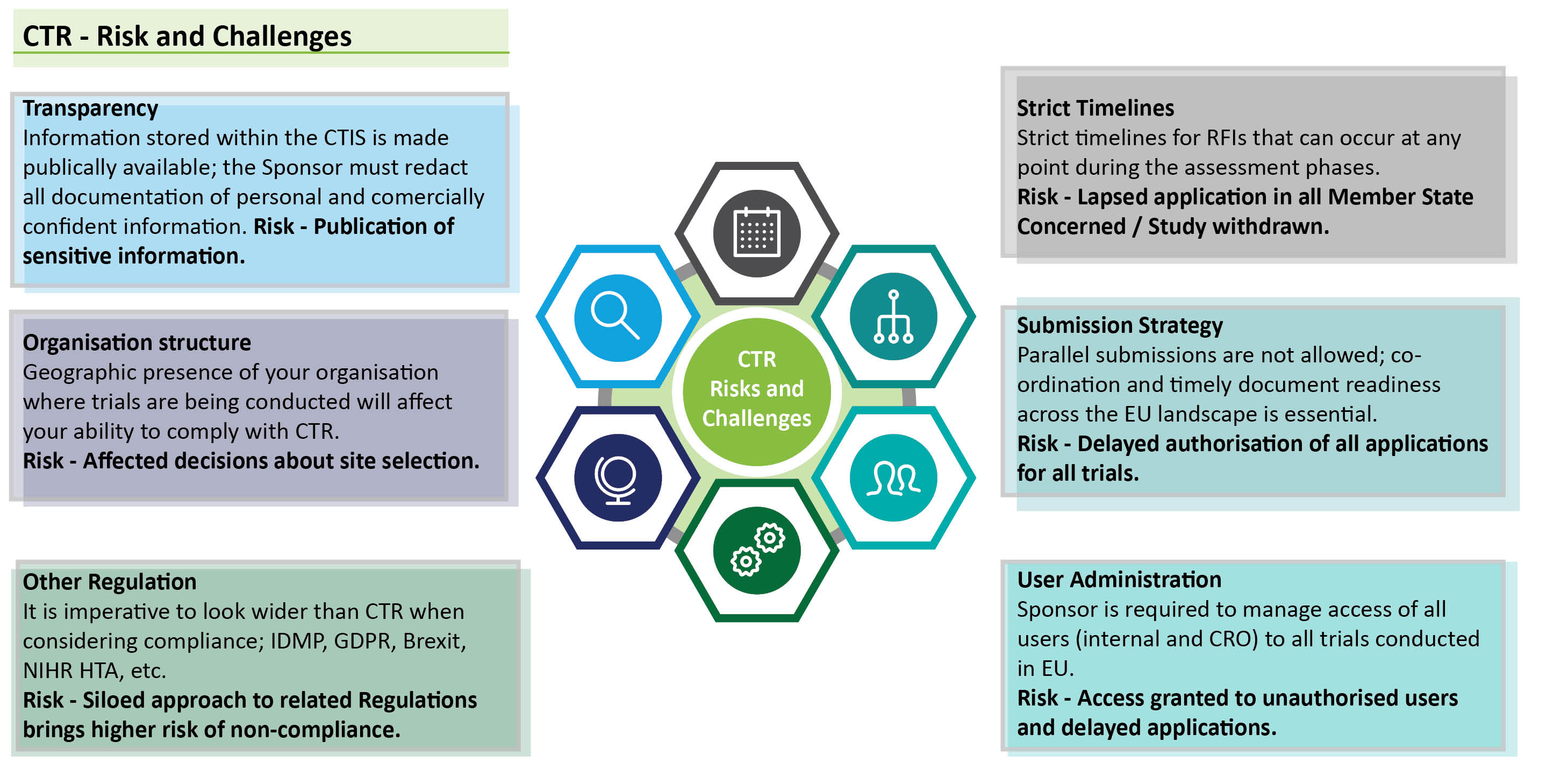
While simplifying the application process and fostering innovation, the CTR also brings about several risks and challenges for organisations:
Some of the more operational challenges which fall under those outlined are:
- ensuring compliance to legal requirements under CTR specifically the need for a European-based legal representative. The CTR requires organisations conducting clinical trials in Europe to have a legal representative based in Europe. This can be a challenge for organisations based outside the EU or for organisations in which the clinical trial sponsor is based outside EU. In our next blog we examine the different options organisations have to tackle this challenge;
- safeguarding personal protective data and commercially sensitive information in published documentation;
- managing user roles across CTIS domains; and
- bridging the transition period between Directive and Regulation.
Conclusion
With these operational challenges in mind, it is important for organisations to prepare well for the upcoming implementation of the Clinical Trials Regulation. In this blog series we will zoom in on these operational challenges in more detail. The next blog will focus on the requirement of a legal representative in Europe and the considerations for organisations.
Legal representation set out in the EU Clinical Trials Regulation
open in new window Read blog 2 here
How can Deloitte support?
We have extensive experience in supporting regulators and top pharmaceutical companies to prepare for new Life Science Regulations, and since 2014 we particularly help prepare for the implementation of the Clinical Trials Regulation. We are skilled with differentiating and setting up regulatory frameworks in challenging and diverse jurisdictions. We pride ourselves in supporting organisations in defining and executing optimal strategies for CTR implementation and ensuring they are and remain responsible businesses. Want to know more? Find our contact details below.